Abstract
Secondary acute myeloid leukemia (sAML) includes a heterogeneous group of diseases evolved from myelodysplasia, myeloproliferative disorders (MDS/ MPN), bone marrow failure syndrome (BMFS) or after exposure to leukemogenic agents such as chemotherapy and/or radiation therapy for malignant hematological (other malignant hematological diseases- OMHD) or solid tumors. sAML has traditionally been considered a devastating disease with inferior outcomes compared to de novo AML, affecting a vulnerable population of heavily pretreated patients. Allogeneic hematopoietic cell transplantation (HCT) is the only potential curative therapy. No systematic large analysis of HCT for sAML is available to study risk factors and outcomes based on prior hematological diseases or solid tumors. Therefore, the Acute Leukemia Working Party of the EBMT has performed a registry study on patients with sAML undergoing HCT. Supplemental data forms requested information on details of previous hematologic (benign or malignant) disease or solid malignancy prior to sAML diagnosis, and cytogenetic data when available.
We studied 4997 patients with sAML who received HCT between year 2000-2016 (prior diagnosis MDS/MPN=3960; OMHD=467; solid tumor=537; BMFS=33). Median age at HCT was 58 years (IQR, 50-64). Median time from diagnosis to HCT was 4.62 months (IQR, 3.18-6.89). Median follow-up of surviving patients was 27 months (IQR, 7.35-60.63). At the time of transplantation, 2625 (52.5%) patients were in CR1 and 2082 patients (41.7%) had active disease. Cytogenetic data at the time of diagnosis of sAML were available in 2016 (40%) of patients (intermediate=1255, adverse 687); 1976 (39.7%) patients received ablative (MAC) and 3005 (60.3%) reduced-intensity conditioning (RIC) regimen (missing=16). Donor sources were matched sibling (MSD) in 1532 (41.7%), unrelated (URD- 9/10 or 10/10) in 2957 (59.2%), other relative (haplo) in 320 (6.4%), and cord blood (CB) in 173 (3.5%). Female donor for male recipients were used in 875 (17.7%) cases and 1097 (24.4%) of patient/ donor pairs were CMV negative pre-HCT. All patients received calcinuerin inhibitor-based GVHD prophylaxis and 2988 (63.9%) received in vivo T-cell depletion (TCD; majority rATG).
Engraftment occurred in 4176 (94.5%) patients. 1317 (28%, 95% CI 26.8 - 29.3) developed acute GVHD (grade II-IV) by day 100. The 2-year cumulative incidence of chronic GVHD, relapse (RI) and non-relapse mortality (NRM) were 33.5% (95% CI, 32-34.9), 33.7% (95% CI, 32.3-35.1) and 27.5% (95% CI, 26.1-28.7), respectively. The Kaplan-Meier estimate of overall survival (OS), leukemia free survival (LFS) and GRFS at 2 years were 44.5% (95% CI, 43-46, 38.8% (95% CI, 37.4-40.3) and 27.2% (95% CI, 25.9-28.6), respectively.
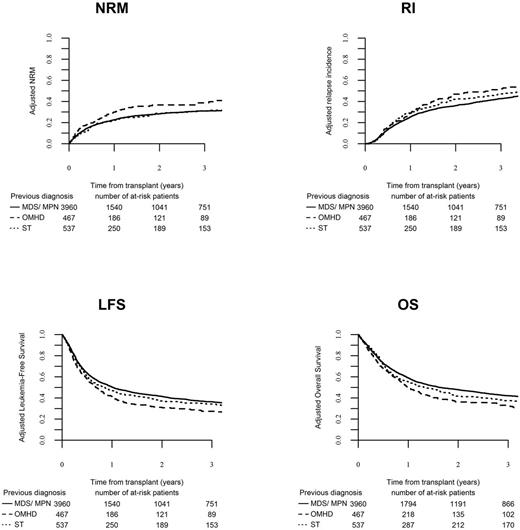
In multivariate analysis adjusted for variables with different distribution among the groups (excluded BMFS group=<1%), patients receiving MAC regimens had decreased RI (HR 0.859, CI 0.761 - 0.97, p=0.01), higher NRM (HR 1.175, CI 1.03 - 1.341, p=0.02) and no differences in OS, LFS and GRFS compared to patients receiving RIC regimens. There was no difference in relapse risk among patients with prior MDS/MPN, OMHD or solid tumor, however prior OMHD was independently associated with higher NRM, inferior OS and LFS. Active disease, adverse cytogenetics, older age, KPS (≤80%), transplant from CB or a haploidentical donor (compared to MSD or URD) and patient CMV-seropositivity were associated with inferior OS and LFS. In-vivo TCD was independently associated with favorable GRFS (HR, 0.885, p=0.007), without any impact on RI, NRM, OS, LFS.
In summary, our registry study is the largest to date to study outcomes for patients who received HCT for sAML. We report that the antecedent hematologic disease (benign or malignant) or solid tumor impacts on outcomes. Patients receiving ablative regimens had a lower relapse risk, and patients transplanted in remission had superior survival. We propose that pre-transplant disease control and post-transplant pre-emptive therapy to decrease relapse risk might further improve outcomes in this high-risk population.
Savani: Jazz Pharmaceuticals: Speakers Bureau. Dreger: Gilead: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; medac: Other: Travel grants; Gilead: Consultancy, Speakers Bureau; medac: Other: Travel grants; medac: Other: Travel grants; medac: Other; medac: Other; medac: Other: Travel grants; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; Gilead: Consultancy, Speakers Bureau; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Riemser: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Riemser: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Riemser: Consultancy, Research Funding; Jansen: Consultancy; Jansen: Consultancy; Riemser: Consultancy, Research Funding; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Jansen: Consultancy; Jansen: Consultancy; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; Jansen: Consultancy; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; medac: Other: Travel grants; medac: Other: Travel grants; medac: Other; medac: Other; Jansen: Consultancy. Ciceri: GSK: Other: B-thalassemia gene therapy was developed by Fondazione Telethon and Ospedale San Raffaele and has been inlicenced by GSK that provides funding for the clinical trial, Research Funding. Schmid: Celgene: Research Funding, Speakers Bureau; MoilMed: Membership on an entity's Board of Directors or advisory committees; Jazz: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding, Speakers Bureau; Novartis: Research Funding, Speakers Bureau. Mohty: Sanofi: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal